Reviving the Lungs and Lactate as a Signalling Molecule
It’s been an interesting week in medicine and personally, which was also why I’m surprised I could write the whole thing in just a day.
It’s been an interesting week in medicine and personally. I’m starting to love the idea of active holidays, which was also why I didn’t do too much writing. I’m surprised I could write the whole thing in just a day.
Also, it's issue number 20! 🎉
Anyway, as the new edition of Nature Medicine came out, I noticed an interesting bunch of studies about reviving organs ex-vivo and using them for transplantations.
Then I came across a tweet that inspired me to look into lactate as a signalling molecule. Until now, it’s been just a metabolic byproduct of exercise for me. I also knew about the “fuel” part, but I barely heard of it being a signalling molecule.
There you go, I think this one should be interesting.
Reviving the Lungs
The achievement of organ transplantations markedly increased human lifespan. It may be considered right up there with vaccines and antibiotics. In recent years, there’s a lot of chatter about constructing new organs. However, there’s a new kid on the block — reviving organs.
The goal of transplant medicine is to increase the pool of organs without increasing the number of people needed to “give” the lungs to someone else…something like that.
One of the possibilities is reconstructing organs from scratch. This is by itself an incredible challenge, but it can be done. When it comes to the lungs, there may be some bigger challenges. When a group of researchers tried to do it, such lungs simply weren’t up for the task. This tells them that it either may not be possible or that it’s too early.
Another viable option uses a slightly different approach. Increasing the pool of lungs for transplant by improving their health ex-vivo. It makes perfect sense if you think about it. Many organs (lungs in this case) are rejected before inserting them into the body of the patients because they are injured, ill or something similar. It would be a game-changer if we could somehow keep the lungs alive, let them heal and then give them to patients.
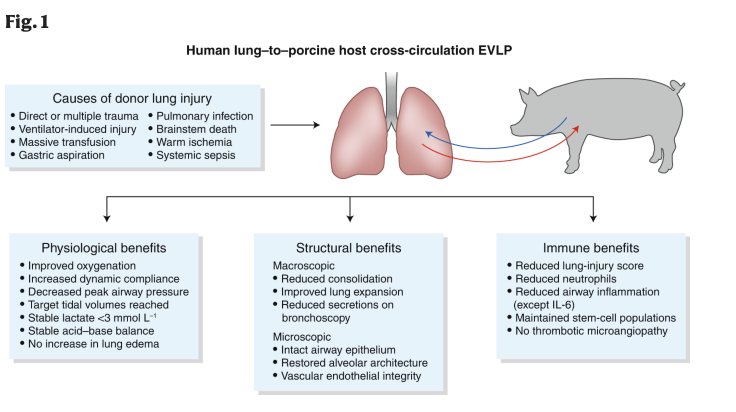
This is exactly what is just starting to happen. Earlier this week a study about reviving dead lungs was published in Nature Medicine. They used a live pig to perfuse the donor lungs with its blood. The lungs don’t just need a ventilator, but also need blood flow to clear any waste substances and so on. The pig kept the lungs alive outside the human body and allowed them to repair themselves. Incredible. This method increased the time the lungs remained “alive” from 6 hours to 4 days. Now imagine what today’s medicine can do in 4 days.
Of course, using pigs isn’t a viable option in the future if these lungs are to get a new and permanent host. But the idea is that we would use live humans instead of pigs. These could even be humans that need the new lungs. They would support the lungs for a short duration they need in the near future.
The discussion about donating organs takes us even further. Anyone who receives a new organ must take immunosuppressants. But they come with a new set of risks as they cause cumulative and irreversible damage. They increase infection, cancer, cholesterol, heart disease, diabetes and kidney failure. Ironically, the very thing that saves a patient is also likely to kill him in the future.
What if we could somehow “teach” our immune system to accept the organs, not suppress it. One of the ideas is to take regulatory T lymphocytes from the patient, grow them in a lab with the cells of a donor and infuse them back into the patient. Another, perhaps an even better option is to take immature white blood cells and develop them into regulatory dendritic cells. The main reason is that they are easier to isolate and faster to grow. But it’s too early to say which is best.
In 2017, a patient in the USA received a new liver and scientists “taught” his immune system to accept the new liver. He went from taking 40 pills per day to taking just one.
Lactate as a Signalling Molecule
Lactate is most known for its function as a waste product that’s produced during strenuous exercise. However, there’s more to it than just that. Throughout the history of lactate research, there have been two main paradigms about it.
The “old” paradigm proposed that it’s a waste product produced during glycolysis and causes muscle fatigue. Fatigue was believed to be the consequence of dissociation of lactic acid to lactate and H+ ions.
However, the “new” paradigm has a completely different perspective. It doesn’t treat lactate as a waste product, but rather as a fuel that’s used by the brain and oxidative muscles. The theory that describes this is called “lactate shuttle theory”. Furthermore, lactate is believed to be produced directly, not with dissociation of lactic acid. As you can see, there’s been a big paradigm shift in the understanding of lactate. Let that be it about lactate in general, but you can read more here.
Lactate shuttle theory brought around a new idea about lactate — as a signalling molecule. Its production and degradation are significantly affected by the concentration of oxygen surrounding the cells. However, because I want to keep this as simple as possible, I won’t be diving deep into its molecular mechanisms. Read more about them here.
I will write more about lactate in 3 systems: adipose tissue, muscles and the brain.
Adipose tissue
Our bodies switch between different types of fuel at different levels of exercise intensity. It’s no secret that fat isn’t used at high exercise intensities as the process of obtaining energy is simply not fast enough. Therefore, our bodies switch to glucose. Of course, with glucose metabolism comes lactate production.
However, this is only one part of the story that doesn’t fully explain why our body switches away from fat as fuel. Its other part is that lactate inhibits lipolysis and thus decrease the amount of fat present in the blood. This happens at lactate threshold — at 4–5 mM. On the other hand, lactate may inhibit glycolysis, which at first sounds strange. But it’s really not if we consider that lactate has to be metabolised. At high concentrations, it’s regulating the processes favouring it being used as fuel.
Muscles
A very interesting thing is going on in the muscles. Lactate causes muscles to grow primarily by two distinct mechanisms. Firstly, it causes differentiation of satellite cells into myocytes. This implies lactate plays an important role in muscle cell growth, repair and maintenance. You’ll find all mechanisms in this paper. Secondly, lactate also plays a role in the secretion of testosterone, which is known to be an anabolic hormone and causing muscle growth. This stems only from experiments in rats, so it’s not completely certain that this mechanism exists in humans. All in all, further study is necessary.
The Brain
Finally, lactate is also present in the brain. The brain is an aerobic organ, whose primary fuel is glucose. In some cases such as starving, it may also switch to using ketone bodies as its fuel. It goes the same for lactate. Additionally, it’s also a signalling molecule.
It arrives in the brain via shuttling from the muscles but is also produced by astrocytes and neurones. Shuttling is happening between these two structures, kind of like between oxidative and glycolytic muscle fibres. The one produced by astrocytes is also used as an energy source by the neurones. The interesting part of signalisation is that lactate activates the neurones of locus ceruleus. An even more interesting part is that it also plays a role in long-term memory formation.
More about lactate and signalling is simply beyond the scope of this newsletter, nor is it its goal. Therefore, I’ll outline some additional resources:




