Artificial Intelligence In Clinical Trials
New standards for designing trials that include AI were introduced in top journals. CONSORT-AI and SPIRIT-AI are the start of a new era in clinical trials.

This month’s issue of Nature Medicine served us with a ton of material from the technology realm. This was also something completely new for me, so I start with the basics and expand.
Traditional randomised controlled trials (RCT or simply clinical trial) are the most important type of study in medicine. They reduce bias and aim to determine the efficacy and safety of new vaccines, drugs, devices and so on (treatments). The results of two or more groups of patients are compared and the results of the treatment are determined. RCTs became common practice in the 1980s, but it took almost 20 years to develop standards of reporting trials - Consolidated Standards of Reporting Trials (CONSORT). CONSORT is a set of guidelines and recommendations for reporting clinical trials. Later, another such guideline was developed called SPIRIT, which stands for Standard Protocol Items: Recommendations for Interventional Trials. These are thus recommendations for designing clinical trial protocols - they are useful before the trial even goes underway.
One of the problems of clinical trials is that they take a long time. Not only do treatments need to undergo 5 phases of clinical trials (0 through 4), but finding appropriate participants is just as hard, if not harder. In 1994, a breast-cancer surgeon needed 5 years to enrol 636 people. Furthermore, most clinical trials fail because of various reasons (read this study for more) and only about 12% of them succeed. All in all, we can say they are inefficient.
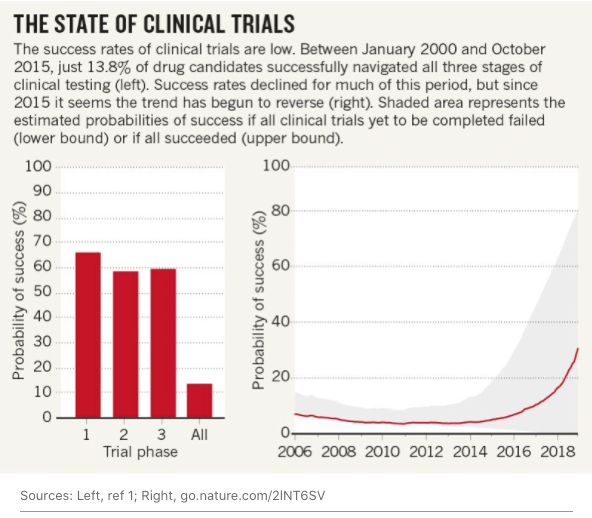
But the probability of success (not the absolute number) in clinical trials is increasing. This is arguably the consequence of AI and a lot of data.
As the recruitment of participants is often the most time and money-consuming part of a clinical trial, improving this part would present a big improvement. As you might imagine, participants are enrolled based on their diagnoses, doctors’ notes and reports. More often than not, some of this is in written form, some in audio and some in images. And even within these subsets, there are differences. For example, a heart attack might be written as a myocardial infarction or MI in different sources. All of this makes it challenging to easily enrol participants.
AI systems are therefore a logical step to take towards improvement. The task of searching for participants in multiple data sets can be the task of natural language processing (NLP), which is a subset of AI.
One of the challenges is the annotation of data, which AI by itself cannot do. According to the outlook in Nature, “there is no such thing as an NLP engine that takes any clinical notes written from any physician and can understand what the notes say”. For now, these systems are not autonomous (see more on autonomous healthcare systems in issue #21).
There’s no question AI will be used ever more often, some say this is a new era of medical research. One of the most effective ways of ensuring a clinical trial is successful is with a great protocol. An interesting start-up called Trials.ai uses NLP to review publicly available data and give researchers feedback on their protocol. This means giving them information on time cost, risk and patient-centricity responding to changes in the protocol. It’s a means of optimising the whole clinical trial.
But as with any new approach, even using AI in clinical trials needs to be proved effective and it must be used in the right way. That’s why researchers need guidelines and checklists for better recruitment and study design. Just as CONSORT and SPIRIT were developed for traditional clinical trials, they received extensions for implementing AI to them. They are called CONSORT-AI and SPIRIT-AI and aim to increase “transparency of study protocols and reporting for randomised clinical trials involving AI.”
CONSORT-AI and SPIRIT-AI are also critical for validating the use of AI in clinical trials. We can achieve validation only if every clinical trial using AI is carried out in the same or similar way and resulting in the same or similar findings. Therefore, the implications of these recommendations span much broader and will affect more than just individual studies, but how these are created and carried out. Plus, as we gather more and more data on adopting AI in clinical trials, these guidelines will change in response to it.




